|
Screenshot of 45 flavours of
nicotine capsules
sold by BAT in Canada
(August 2022) |
Earlier
this spring, we reported on hardware and cosmetic changes to the VUSE vaping device sold by British American Tobacco/Imperial Tobacco Canada Ltd (BAT-ITL) was marketing its VUSE brand ePOD vaping device. Among these developments were the ability of consumers (and the company?) to monitor device use and location through internet connections, increased personalization of nicotine delivery and cosmetic elements and the expansion of flagship stores.
This post reports on the further evolution of the company's marketing activities: its continuously expanding flavour range and its introduction of strength bands for nicotine, similar to those in place for its cigarettes.
Background
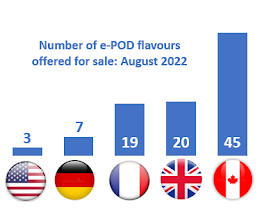
BAT-ITL currently only sells the cartridge-based ePOD line of vaping devices in Canada. The ePOD device was introduced to Canada at the beginning of 2019 with 5 flavour offerings. In January 2020, the number of flavours offered had grown to 8, increasing to 22 flavours in January 2021 and 30 flavours in January 2022. This month (August 2022) there are more than 40 flavours available for nicotine liquids.
The ePod capsules were originally sold in Canada in 3 nicotine strengths (1.6%, 3% and 5%), but for a year after Health Canada imposed a ceiling on nicotine concentrations in 2021 only one strength (18 mg/ml, 1.6%) was available, in addition to nicotine-free. Today, as discussed below, three nicotine levels are available.
BAT does not sell the same vaping products in all markets, reflecting both regulatory and market differences, as seen in their offerings in its 5 largest vaping markets : Canada, France, Germany, the UK and the USA.
BAT sells fewer devices in Canada. In the
United Kingdom and
France it also sells the eTank and ePen, and has recently introduced the disposable VUSE GO/PUFF. In the United States, BAT's subsidiary
sells the ePOD under the name ALTO as well as three cigalike products (SOLO, CIRO and VIBE).
BAT sells higher nicotine in Canada than in Europe. In
France and
the UK, nicotine concentration is capped at 20 mg/ml as it is in Canada. In those countries, however, BAT offers some much lower nicotine strengths than it does in Canada (e.g. 3 mg/ml and 6 mg/ml) as well as offering 12 mg/ml and 18 mg/ml as it does in Canada and Germany. There is no maximum nicotine concentration in the USA, and the range of nicotine concentrations for ePOD-ALTO capsules include higher but not lower strengths than are sold in Canada (e.g. 18, 24 and 50 mg/ml).
The continuous expansion of flavours
This spring, BAT-ITL passed the '31 Flavour' threshold in Canada, now selling more flavours than leading ice-cream chains. As noted above, the number of flavours has doubled over the past 18 months, and sextupled since the year before.
Most of the new flavours introduced this summer are in the flavour categories that Health Canada has indicated would be exempted from any flavouring restrictions: tobacco-flavour and mint-menthol. They are also the flavours that are subject to fewer provincial flavour restrictions.
Since May 2022, the number of mint-menthol flavours for ePOD capsules has grown from 6 to 13, and the number of tobacco flavours has grown from 7 to 13.
6 mint-flavoured ePOD liquids listed in May 2022:
13 mint-flavoured ePOD liquids listed in August 2022:
This marketing strategy creates several concerns for public health authorities:
Continued taste tests help BAT turn experimenters into nicotine addicts. Researchers (
including in Canada [2]) have established the brief trajectory from a first cigarette puff to medically-defined dependence. If, as we should expect, the milestones to addiction are similar for vaping products, BAT knows that it needs to encourage users to move beyond a first experimental puff to subsequent experiments and regular use. The strategy based on continued experimentation needs a continuous supply of "new" flavours.
The focus on flavours interferes with awareness of risks. Directing attention to flavour 'notes' and 'intensity' serves to increase the importance of flavours to the user, and reinforce the pleasurable aspects of the vaping experience. This serves to downplay the inherent health risks of the product.
Flavourings increase the risks of vaping. In addition to their role in attracting new users and establishing addiction,
flavourings also make vaping more dangerous by increasing the exposure of users to harmful chemicals and particulates.
BAT's flavour promotions include the development of a lexicon to discuss flavour experiences. In addition to the the flavour card shown here, the company offers an interactive VUSE IQ on line and in its stores to guide flavour selections. Nicotine strength bands that echo cigarette marketing
This summer, the company began marketing their e-liquids in Canada in three bands of nicotine strength, in addition to nicotine-free. The salted 1.6% formulation is now labelled "balanced", against which "mellow" and "bold" strength versions are compared.
*
BOLD liquids (also sold as V-ULTRA) are made with slightly more nicotine (20 mg/ml instead of 18 mg/ml), and use free-base nicotine which
has a stronger throat impact and is less easy to inhale [3]. BAT uses
blog posts,
social media and direct mail to promote this as
"a bold nicotine experience and an intense sensorial kick."
* MELLOW liquids have one-third less salted nicotine than balanced (12 mg/ml or 1.1%). Web promotions, social media and direct mail promote this as "a Mel-low nicotine experience" - "high on flavour, low on nicotine".
This strategy echoes the presentation of BAT cigarettes, such as John Player, as 'mellow', 'smooth', 'rich' and 'bold'. Although the company maintains these descriptors relate to taste, and not nicotine strength, they originate in the era when 'light'/'mellow' cigarettes were promoted as being less harmful or addictive.
Coming soon to Canada? BAT's VUSE disposables:
Implications for regulators
BAT's marketing strategy may be flying below the public health radar.
This year BAT has launched new vaping retail outlets, has started connecting vaping devices to monitoring systems, has added jewelry and other cosmetic features to its vaping devices and has greatly expanded the range of flavours and marketing techniques to promote them. Each of these measures appears designed to increase the use of vaping products for pleasure (not cessation).
None of their actions this year has elicited a response from any of Canada's 14 health regulators. No government has disclosed any system in place to monitor these market changes. (The regulations proposed by Health Canada this summer
do not include any requirements to report on promotional activities.) Unlike most other countries, there are no requirements for BAT to notify Canadian health authorities before selling vaping liquids and devices, allowing it to use Canada as a proving ground for its global brands.
Proposals to restrict flavourings,
currently under review in Canada and elsewhere, are largely based on research that treats "tobacco flavour" and "menthol flavour" as two flavourings and not two flavour ranges encompassing dozens of flavours. BAT's launch of 26 flavours in these two categories suggests that it is poised to blunt the impact of federal restrictions originally considered to restrict the number of flavourings.
References
[1] BAT Capital Markets Day 2019. Step-Changing New Categories. A very significant growth opportunity. https://www.bat.com/group/sites/UK__9ZTFCM.nsf/vwPagesWebLive/DOBA7JRL/$FILE/Step-Changing_New_Categories_-_A_very_significant_growth_opportunity.pdf?openelement
[2] André Gervais, Jennifer O’Loughlin, Garbis Meshefedjian, Christina Bancej, Michèle Tremblay
Milestones in the natural course of onset of cigarette use among adolescents. CMAJ • August 1, 2006
[3] SohaTalih, Rola Salman, Rachel El‑Hage, Nareg Karaoghlanian, Ahmad El‑Hellani, Najat Saliba and Alan Shihadeh. Effect of free‑base and protonated nicotine on nicotine yield from electronic cigarettes with varying power and liquid vehicle. Nature. (2020) 10:16263
[4] BAT. News Release. Half-Year Report to 30 June 2022. 27 July 2022. https://www.bat.com/group/sites/UK__9D9KCY.nsf/vwPagesWebLive/DOCGKBGW