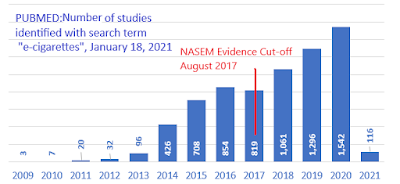
This week marks three years since the US National Academies of Sciences, Engineering and Medicine (NASEM) released a report commissioned by the U.S. FDA on the "Public Health Consequences of E-cigarettes." Since then, this report has often been cited as a definitive source for questions about the safety of e-cigarettes, and is a primary source for Health Canada's encouragement to smokers to switch to e-cigarettes.
Thirty-six months later, however, the conclusions in this report are increasingly stale-dated. The panel of scientists who wrote the report looked only at evidence available before August 2017 - and for some key issues (like heart and lung diseases) there were virtually no published studies available for them to consider.
Since then there has been a marked growth in scientific evidence about these products. Of the approximately 7,000 studies on e-cigarettes listed in the U.S. National Library of Medicine, more than half (57%) were published after 2017. From today's vantage point, the NASEM report tells less than half the story.
In general, these more recent studies have provided evidence on health hazards where there previously had been none and have strengthened those links that had already been identified. Newer studies have also dashed the hopes inferred from the NASEM report that these products could be effective for population-wide smoking cessation.
1. The NASEM report, even in 2018, did not provide all we needed to know to regulate e-cigarettes
The NASEM report was a weighty tome of nearly 800 pages. Perhaps because of its weight, many mistakenly considered it encyclopaedic, containing all the information one would need to know about e-cigarettes.
But there were key aspects of the e-cigarette issue which were not addressed by this report. Notably it did not consider nor predict that there would be an explosion of vaping among young people in Canada and the United States. ( By 2019, there were 700,000 vapers in Canada under the age of 25, one-half of all vapers).
The report did not analyze the behaviour of tobacco and vaping industries. It did not predict that the tobacco industry would come to dominate the vaping market, or consider the evidence that would predict the impact of their doing so. It did not analyze the impact of the industry developing nicotine salt products, or anticipate that these higher-nicotine versions would soon domiate the market and accelerate the addiction of (mostly young) people to nicotine.
It did not predict or analyze the imapct of e-cigarettes becoming a consumer product used for recreational drug purposes as much as to avoid smoking cigarettes, or that the marketing of these as consumer products would result in their not proving effective at helping people quit smoking. It dismissed the likelihood that the e-cigarette could increase harm, and did not forecast that the prevalence of nicotine use among young people would increase dramatically as a result.
2. The NASEM report has been misinterpreted as endorsing e-cigarettes
Perhaps spurred on by the hope that e-cigarettes would be an effective mass smoking cessation aid, some seized on just a few of the NASEM conclusions that seemed to suggest some benefit of
e-cigarettes to guide policy, but ignored the others. A careful reading of the whole report and the analysis behind its 47 conclusions would have led to a more cautious approach.
3. Five adverse health outcomes where NASEM concluded "no available evidence" in 2017: an update
In September 2020, based on evidence available up to April 2019, the European Union's Scientific Committee on Health, Environmental and Emerging Risks (SCHEER) found evidence linking e-cigarette use to respiratory disease, heart disease, to adolescent smoking and to addiction.
In April 2020, the Trimbos Institute reported to the Dutch government that "Newer studies provide more and more indications that the use of an e-cigarette could lead to damage to the respiratory tract and the cardiovascular system."
Reviews on the impact of e-cigarettes on tobacco use conducted for the government of Australia by that country's National Centre for Epidemiology and Public Health concluded that e-cigarettes did not help people quit smoking, but did increase the probability of a young person starting to smoke. In its own review, the Australian Ministry of Health felt that current evidence on the direct health harms associated with e-cigarettes justified taking a precautionary approach.
 |
| Health Canada's advice on the health risks of vaping does not identify risks other than those related to nicotine |